- Ultrasonic Degassing Of Molten Metals up to 2.300°C And Molten Glass
- Monday - Friday : 09:00 - 19:00
- Sialon Ceramics Sweden AB, Lila Kungsgatan 2, SE-411 08 Göteborg, Sweden
Molten Glass Energy Savings
Molten glass energy savings
We double the bubble size and reduce the temperature (Stoke's Law)
Ultrasonic effect of bubble size on refining rate
Ultrasonic assisted molten glass refining can potentially be used to remove gas bubbles from molten glass at substantially lower temperatures than the standard process and, very importantly, without the need to increase processing times. As the molten glass refining process is highly energy-intensive, reducing the refining temperature provides significant energy savings. Here we show that ultrasonic refining has the potential to reduce total energy costs by up to 20%.
Glass melting and refining
Glas manufacture is a highly energy-intensive process which involves both melting and refining. Melting refers to controlling the chemical reactions that occur in the formation of the glass from raw materials, and refining refers to the process of removing bubbles from the molten glass that is formed.
Melting soda-lime glasses is achieved at temperatures of around 1,300 °C while refining requires significantly higher temperatures of about 1,450 °C. At this higher temperature, the viscosity of the molten glass is sufficiently low to allow bubbles in the glass to rise to the surface.
Raising the temperature from 1,300 °C to the refining temperature of 1,450 °C consumes around 40% of the total energy used in the complete melting and refining process. Typically the combined melting and refining processes take approximately 24 hours and so consumes a considerable amount of energy.
There is a strong correlation between bubble size, refining temperature, and time. Simply expressed, larger bubbles rise faster than smaller bubbles; higher refining temperatures reduce the viscosity of the molten glass; bubbles rise more quickly when the viscosity is lower. Thus, to improve the efficiency of the refining process, we would like to increase the bubble diameter and/or reduce the viscosity of the molten glass. As we shall see, ultrasonic refining allows us to increase bubble size and remove bubbles efficiently without the need to raise the temperature of the molten glass.
The effect of bubble size on refining rate
More formally, if the gas bubbles were to coalesce, then, according to Stokes Law, the same refining rate could be maintained at higher viscosities. Stokes law expresses the force F of a stationary sphere of radius R suspended in a fluid with a viscosity of η moving with a relative velocity v.
F = 6 π η R v
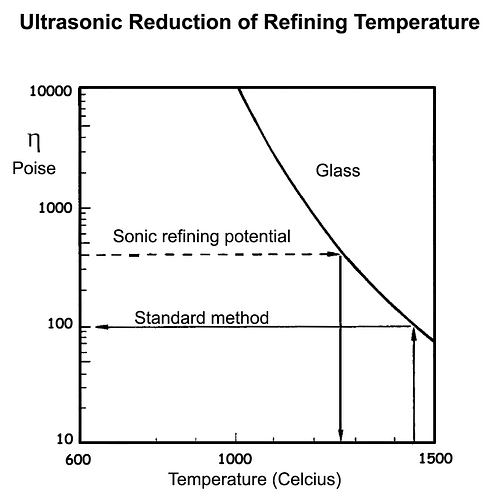
We can use this relationship to show that doubling the bubble diameter from say 0.4 mm 0.8 mm would theoretically allow us to achieve the same refining rate in a molten glass with a viscosity of 400 Pa as would occur with the smaller bubbles and a viscosity of 100 Pa. Note that 0.4 mm is the typically the smallest bubble diameter that would rise through a depth of one meter of molten glass in around 16 hours, a rate of 20 micrometres/second.

The relationship is apparent from the diagram, which shows viscosity versus temperature for a typical commercial glass. At 1,450 °C, the viscosity is 100 Pa, while at 1,280 °C it has been reduced to 400 Pa. From Stokes law, the rate of rising of a 0.4 mm bubble at a viscosity of 100 Pa is the same as that of a 0.8 mm bubble at 400 Pa.
In other words, by doubling the size of the bubble, we could achieve the same refining rate at a reduced temperature of around 1250 °C. Typically, this would save 20% of the total energy cost of the process.
While this analysis suggests that we can make substantial energy savings to the refining process by increasing the bubble diameter in molten glass, can we achieve this practically?
viscosity versus temperature
Forcing bubble coalescence using ultrasonic energy
The latest advances in ultrasonic refining mean that we can now use ultrasonic energy to increase bubble size during glass refining. While there are multiple processes involved, the dominant one is cavitation.
Cavitation is a high energy process which rapidly changes the pressure in the molten glass creating low-pressure cavities or voids. The voids provide nucleating centres into which dissolved gasses and bubbles diffuse and accumulate. Effectively, small bubbles coalesce forming larger bubbles, which, as we have shown, rise more rapidly to the surface where they dissipate.
Conclusion
Increasing bubble size during glass refining using ultrasonic energy means we can achieve standard refining times without the need to reduce the viscosity of the glass melt by increasing its temperature following the glass melting process. This means we have the choice of reducing processing times or reducing the energy used in the process.
While potential energy saving will vary across the industry and will depend on many factors, including the glass industry sector and quality requirements, potential energy savings of up to 20% are available. This energy-saving offers a substantial potential benefit for this highly energy-intensive industry.
